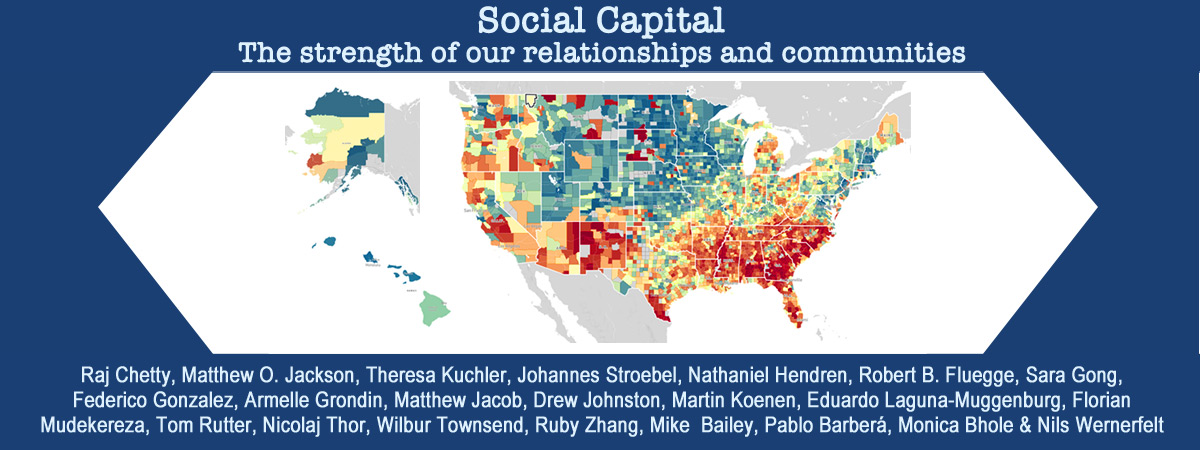
Chemical and physical properties of surfaces play crucial roles in catalysis, environmental processes, semiconductor technology and many other applications. The structure of a real surface is often imperfect with a variety of defects. These include geometric defects such as step edges, kinks and other lattice mismatches, as well as chemical defects such as lattice vacancies and interstitial particles, which can dominate the chemical properties at the active surface.
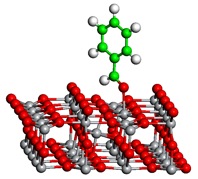 We study the reactivity of rutile TiO2(110) surfaces, a material which is often used as a support for heterogeneous catalysts and also a catalyst itself. The major point defects in TiO2(110) are bulk and surface oxygen vacancies, as well as bulk Ti interstitial atoms. Bulk vacancies and interstitials are typically considered to be static in nature, but we recently obtained strong experimental evidence that Ti interstitials from the bulk can migrate out to the surface and react with aldehydes and ketons, leading to a coupling to alkenes (McMurry chemistry) and the formation of new TiO2 particles on the surface. Currently we are performing extensive theoretical investigations of the various effects of defects on the bonding and reactivity of carbonyls. By computing possible reaction intermediates, pathways and energy barriers, we can unambiguously determine the underlying reaction mechanisms and the role of the defects.
We study the reactivity of rutile TiO2(110) surfaces, a material which is often used as a support for heterogeneous catalysts and also a catalyst itself. The major point defects in TiO2(110) are bulk and surface oxygen vacancies, as well as bulk Ti interstitial atoms. Bulk vacancies and interstitials are typically considered to be static in nature, but we recently obtained strong experimental evidence that Ti interstitials from the bulk can migrate out to the surface and react with aldehydes and ketons, leading to a coupling to alkenes (McMurry chemistry) and the formation of new TiO2 particles on the surface. Currently we are performing extensive theoretical investigations of the various effects of defects on the bonding and reactivity of carbonyls. By computing possible reaction intermediates, pathways and energy barriers, we can unambiguously determine the underlying reaction mechanisms and the role of the defects.
Figure: Density Functional Theory optimized structures of an adsorption configuration and of a reaction intermediate during the formation of stilbene from benzaldehyde (carbon: green, oxygen: red, titanium: grey, hydrogen: white).
Faculty:
Prof. C. Friend